Polymer Dispersions

WACKER started manufacturing polymer dispersions on an industrial scale in 1938 and has developed its integrated production process ever since. Starting from VAM (vinyl acetate monomer) as the major raw material, WACKER produces a variety of polymer dispersions including PVAc homopolymers and VAE (vinyl acetate-ethylene) co- and -terpolymers.

Vinyl acetate-ethylene (VAE) is a combination of ethylene and vinyl acetate - two monomers possessing special properties as e.g. toughness (vinyl acetate) and flexibility (ethylene). Polymerization leads to VAE copolymers. VAE copolymers form the basis for engineering different properties in the individual end application.
VAE dispersions are based on the polar monomer vinyl acetate and the hydrophobic monomer ethylene. They are produced by emulsion polymerization in an aqueous environment, without the addition of organic solvents. WACKER markets VAE dispersions under the trade names VINNAPAS® and ETONIS®.
Main Characteristics:
The interaction between vinyl acetate and ethylene monomers provides VAE dispersions with an outstanding range of technical performance benefits, along with environmental advantages, at an attractive cost-in-use ratio. For example, flexibility, adhesion and water resistance can be adjusted as needed by varying the monomer composition.
Main Benefits:
Very good adhesion to a wide range of substrates, including wood, paper, metal, plastics and concrete
- Broad range of available glass transition temperatures (Tg)
- The VAE formulation strategy eliminates the need to add plasticizers
- Good film-forming properties at low temperatures and without additional coalescing agents
- Excellent filler acceptance
- Excellent cohesion
- Heat and fire resistance over a wide range
- All modern dispersions are produced without added APEOs
Main Application Areas:
- Adhesives
- Architectural coatings
- Construction
- Nonwovens
- Carpets
- Paper
- Caulks and sealants
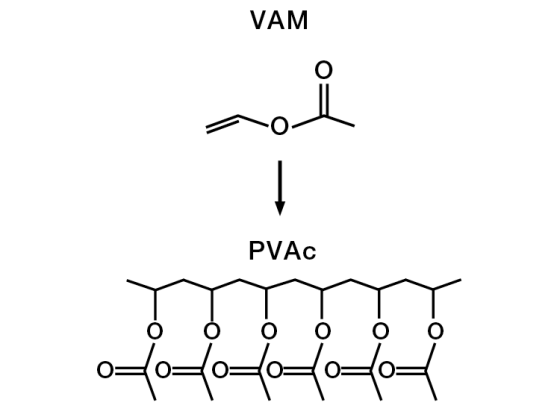
Vinyl acetate monomer (VAM) is an important starting material in the production of dispersions and dispersible polymer powders.
WACKER markets PVAc dispersions under the trade names VINNAPAS® and ETONIS®.
Main Characteristics:
PVAc dispersions are polar, hydrophilic homopolymers. They are used as binders and polymeric modifiers and promote strong adhesion and cohesion.
Main Benefits:
- Very good cohesion and adhesion to cellulosic substrates
- High heat resistance
- High Tg
Main Application Areas:
- Adhesives
- Construction
- Concrete modification
WACKER produces dispersions based on VC co- and terpolymers. They are marketed under the trade names VINNAPAS® and VINNOL®.
Main Characteristics:
Due to the self-extinguishing properties of vinyl chloride polymers, such dispersions exhibit inherent fire retardation without the addition of fire retardants or additives. Furthermore, they provide excellent hydrophobicity.
Main Benefits:
- Flame retardancy
- Excellent adhesion to textile fibers
- Abrasion resistance
- Improved hydrophobicity
- Variety of hands
- Controlled absorbency
- Wet and dry tensile strength
Main Application Areas:
- Nonwovens
- Textile finishing
- Architectural coatings

The composite technology combines the benefits of inorganic material and organic polymer and silane chemistry: The hard shell (acrylic copolymers) provides e.g. toughness for excellent dirt-pick up resistance. The soft core (silica particle) ensures e.g. outstanding film-forming properties, even without the use of coalescing agents.
Under the trade names PRIMIS® and VINNAPAS®, WACKER offers a range of innovative acrylic-based dispersions.
Main Characteristics:
WACKER offers high-performance, specialty dispersions based on acrylic technologies. Here, WACKER combines its expertise in polymers with that in silicon chemistry to develop products with unique application profiles.
Main Benefits:
- Oil resistance combined with hydrophobicity
- Excellent film formation even at low temperatures and without film-forming agents
- Very good hydrophobicity
- Excellent mechanical behavior
- Very good dirt pick-up resistance
- Improved abrasion resistance
Applications:
- Architectural coatings
- Plasters / renders
- Primers
- Waterproofing membranes
- Tile adhesives



