Our system has noticed that you are based in , but the current country setting is . Select country

Transdermal drug delivery systems: silicone adhesives for transdermal patches
Our study with more than 20 active ingredients shows that silicone gel adhesives enable targeted drug release – rapid, delayed or constant.
Our study reveals how silicone gel adhesives are transforming the release of active ingredients – in cosmetics, nutraceuticals and pharmaceuticals. Release properties can be tailored: steady release, burst release or long-term release.
When it comes to hydrophobic actives, we developed silicone adhesives allowing good mixability and superior release rates, outshining commercially available products.
Study results: silicone gel adhesives for transdermal patches
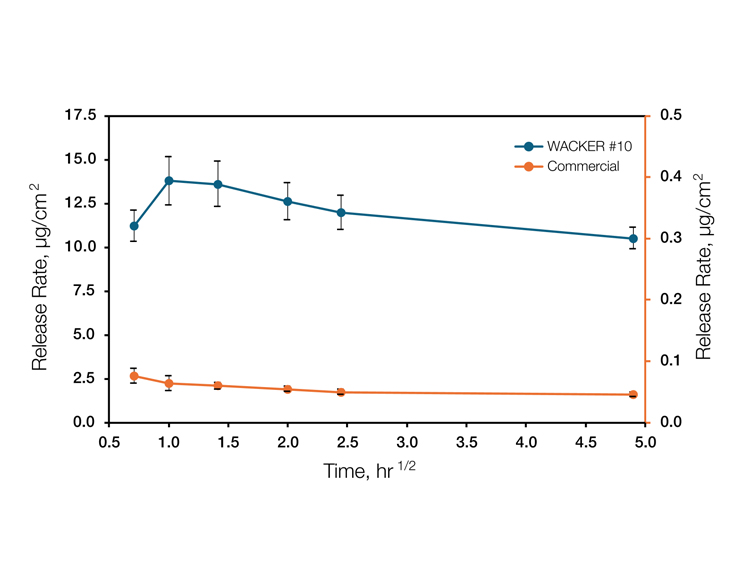
Here is a summary of our study:
Study focus:
Key findings
Download the complete study.
Review and evaluation of transdermal drug delivery systems
For the benchmark studies on transdermal drug delivery systems using silicone gel adhesives, our researchers from the WACKER Health Care Competence Center in Ann Arbor, USA, examined the following questions:
The team used a silicone layer that is a maximum 300 micrometers thin. The Franz diffusion cell methodology was used to evaluate the skin penetration and the release of more than 20 active ingredients.
“We were able to demonstrate an above-average release of 20 active ingredients from the silicone gel matrix. Moreover, we could show that silicone gel adhesives are suitable for the development of novel release profiles.”

Optimum release for medical patches and beauty pads
In many applications, the steady release over time of the active ingredients is important. Silicone gels are ideal for such applications and are therefore a promising alternative to existing pressure-sensitive adhesives.
Latest trends: administering actives through the skin

Transdermal patches deliver active ingredients such as hormones steadily over time.
Administering a cosmetic, nutraceutical, or pharmaceutical active ingredient by means of a transdermal patch or topical ways has several advantages:
Patch designs: drug-in-adhesive and reservoir patch design
Today’s dressings can do more than just cover and protect wounds. Equipped as transdermal patches, they have functional layers that can store and release different kinds of active ingredients.
This opens up new possibilities for:
Predominant types of transdermal patch designs:
Silicone gel adhesives: skin-friendly drug delivery systems

Sample of SILPURAN® medical silicone adhesive, coated to PU laminate
WACKER has developed a process for systematically manufacturing and testing silicone adhesives for transdermal drug delivery systems. For the tests, we used silicone gel adhesives from the SILPURAN® product portfolio.
They are:
WACKER’s SILPURAN® silicone gel adhesives are categorized as addition-curing room-temperature vulcanizing silicones (RTV silicones). They consist of two components: the silicone rubber base and the crosslinker.
SILPURAN® silicone gels
Tested active ingredients: 20 non-prescription and cosmetic applications
Transdermal patches are increasingly being used for non-prescription and cosmetic applications. WACKER therefore focused on developing silicone gels that can be used for administering the most common substances.
The goal was to achieve maximum and tailored release properties while maintaining the structural integrity of the adhesive coating. The active compound was embedded to a silicone gel matrix that is a maximum 300 micrometers thin.
More than 20 active ingredients have been tested:
We evaluated:

From raw material to analysis: process for manufacturing and evaluating silicone adhesives for transdermal therapeutic systems.
Download the complete study.
Transdermal drug delivery systems
DownloadTesting methods: release rates of active ingredients
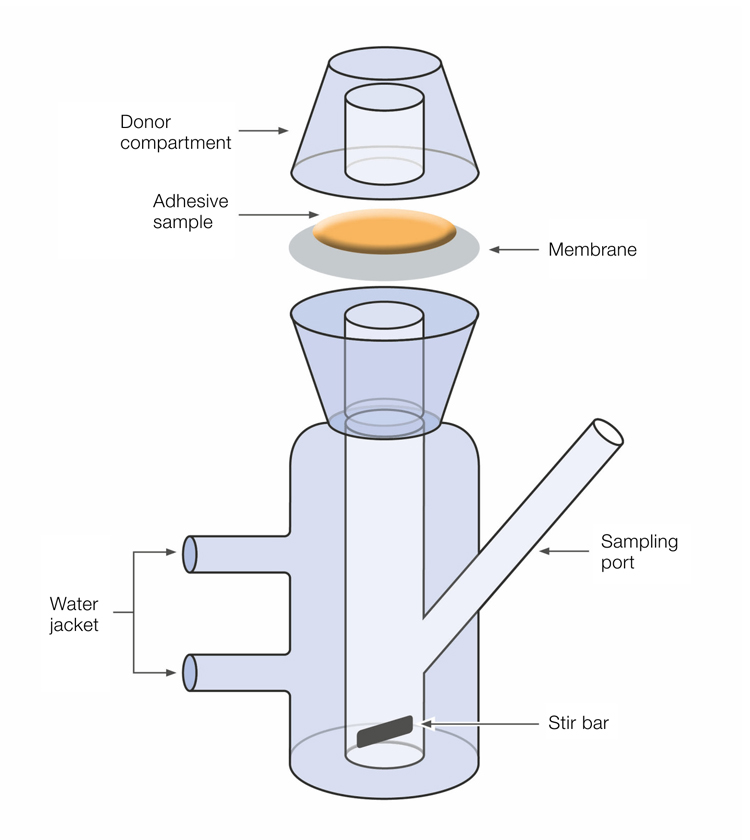
The Franz diffusion cell method is often used to study skin penetration and the release of active ingredients from various formulations – including transdermal patches.
The release rates were quantified using either the Franz cell methodology or the orbital shaker method.
The Franz diffusion cell system is designed to simulate the behavior of active compounds and formulations when applied to the skin.
In this system, the test sample is placed in contact with a membrane, and the rate of transfer is determined by collecting the permeate on the opposite side of the membrane.
For limited penetration studies, we employed the Strat-M® membrane for transdermal diffusion testing.
Download the complete study.
Transdermal drug delivery systems
Download“Patches coated with silicone gel adhesives can be customized to the intended rate of release of the active ingredient. Thus, they can be tailored to the treatment goals of the respective application. What’s more, silicone gels display good skin compatibility.”

Download the complete study here!
Download
Related:





