PLASMITEC®: WACKER Introduces Its Plasmid DNA Production Platform
Munich / San Diego, Jan 17, 2022
WACKER is offering its platform for developing and manufacturing plasmid DNA (pDNA) under the brand name PLASMITEC®. The proprietary technology platform relies on decades of experience in pDNA manufacturing at the newly acquired San Diego site. It can be used for applications in research, clinical development and commercial manufacturing. pDNA is the basis for a wide range of innovative therapeutic agents – a market in which demand continues to rise.

Plasmids – important tools for manufacturing biopharmaceutical agents – are tiny rings of double-stranded DNA that can replicate independently of the bacterial chromosome. Demand for pDNA has grown sharply of late as part of efforts to develop and manufacture mRNA-based vaccines for COVID-19. While pDNA is a critical raw material for mRNA production, it has many other therapeutic applications. pDNA serves as the starting material for producing viral vectors, for instance, which are used in gene editing and gene therapies. Current studies anticipate that the market for manufacturing pDNA will grow by 22 percent annually between now and 2030, reaching €1.9 billion in 2030.
Wacker Biotech is a contract development and manufacturing organization (CDMO) bundling the biopharmaceuticals activities of the WACKER Group. Wacker Biotech produces pDNA for its customers at its site in San Diego, USA. The development and manufacturing platform is available under the brand name PLASMITEC®. Starting with the pDNA sequence, the platform offers a complete service capability – from selecting and generating productive strains, up to and including GMP-compliant manufacturing. “With PLASMITEC®, we are able to rapidly produce plasmids in both small and large scale for our customers for all phases of clinical development and commercial supply. Our pDNA platform combines comprehensive expertise with years of experience manufacturing pDNA to GMP standards,” says Guido Seidel, who is responsible for the biopharmaceuticals business at WACKER.
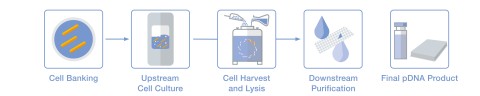
The first step in producing plasmid DNA is the generation of cell banks from a starting plasmid (see illustration). This is followed by fermentation in larger reactors, in which the actual production of plasmid DNA takes place. The resulting cell paste is then harvested. Next, the plasmid is extracted from the cell paste using a scalable alkaline lysis process – a key production step that also makes PLASMITEC® practical for large-scale applications. In a multistage purification process, unwanted components are removed from the extracted pDNA molecules, which are then purified and concentrated. The final step is to fill the finished pDNA into customer-specified containers.
Wacker Biotech US Inc. in San Diego is equipped with a 43 L single-use reactor and a 650 L stainless steel fermenter for production, including scalable cell lysis. Primary recovery and downstream capacities are available for a variety of customer needs and applications. The facilities have been certified as GMP-compliant. The roughly 50 employees at the site have over 18 years of experience and comprehensive expertise in the field of pDNA manufacturing under GMP conditions.
Since WACKER acquired the biotech site in February 2021, the Group has invested some US$3 million in San Diego. That investment has gone toward modernizing the automation technology of the production plants, among other projects. “Our innovative technologies and quality systems, the expertise of our employees and our ultramodern production facilities put us in a position to meet rising market demand for pDNA,” says Philippe Cronet, general manager of Wacker Biotech US Inc.
About Wacker Biotech
Wacker Biotech GmbH, Wacker Biotech B.V. and Wacker Biotech US Inc. are full-service contract manufacturers of therapeutic proteins, plasmid DNA (pDNA), live microbial products (LMPs) and vaccines based on microbial systems. The company’s portfolio extends from strain/process development, through analytical testing, to production for clinical and commercial applications. Wacker Biotech maintains three GMP-compliant (Good Manufacturing Practice), FDA- and EMA-certified production plants at its German sites in Jena and Halle as well as in Amsterdam in the Netherlands. The company’s most recent acquisition is its San Diego site (Wacker Biotech US Inc.), added in February 2021. Wacker Biotech GmbH, Wacker Biotech B.V. and Wacker Biotech US Inc. are wholly owned subsidiaries of the Munich-based WACKER Group. More information is available at: www.wacker.com/biologics
Live Webinar at Advanced Therapies Week 2022
We will introduce our PLASMITEC® pDNA production platform in a virtual presentation at Advanced Therapies Week 2022.
- ‘Wacker Biotech – The Microbial CDMO for Advanced Biotherapeutics’
- Speaker: Peter Danforth, Business Development Manager, Wacker Biotech GmbH
- January 27, 2022, 3:50 p.m. – 4:05 p.m., EST
- Register here.: http://wch.ag/FGhGi
Press Picture
Graphic: Production of plasmid DNA
Production of plasmid DNA in five steps: from cell bank production and cell culture preparation to cell harvesting, lysis and purification, to the finished pDNA product (illustration: WACKER).
Download picture (JPG, 640 KB)Wacker Biotech US Inc. in San Diego
As a contract manufacturer, Wacker Biotech US Inc. in San Diego operates a specialized fermentation line for manufacturing and purifying plasmid DNA (photo: WACKER)
Download picture (JPG, 453 KB)Contact

Wacker Chemie AG
Media Relations
Manuela Dollinger
Tel. +49 89 6279-1629
Send message
Download
Press Information
(PDF | 318 KB)






