Expert Vaccine Manufacturing
Wacker Biotech is your CDMO partner of choice for the process development and manufacturing of a diverse array of vaccines, ranging from live, attenuated, inactivated and (conjugated) polysaccharide vaccines, to protein and mRNA-based vaccines.
Vaccine Innovation and Experience

Wacker Biotech has more than 30 years of experience manufacturing conventional vaccines using microbial hosts for various infectious diseases. We are proud to have partnered with clients to bring several commercial programs to market that continue to protect populations worldwide.
More recently, after years of research, we have developed the expertise to produce next-generation mRNA-based vaccines and have built a center of competence in Halle, Germany with four new dedicated lines for mRNA production. The state-of-the-art center was partially funded by the German Government as part of its Pandemic Preparedness program.
Vaccine Capabilities
Vaccine Types
Experience with Diverse Bacterial Strains
Key Benefits of Wacker Biotech Vaccine Services
Our Conjugated Polysaccharide Vaccine Production Process
Click orange dots to learn more about each process step
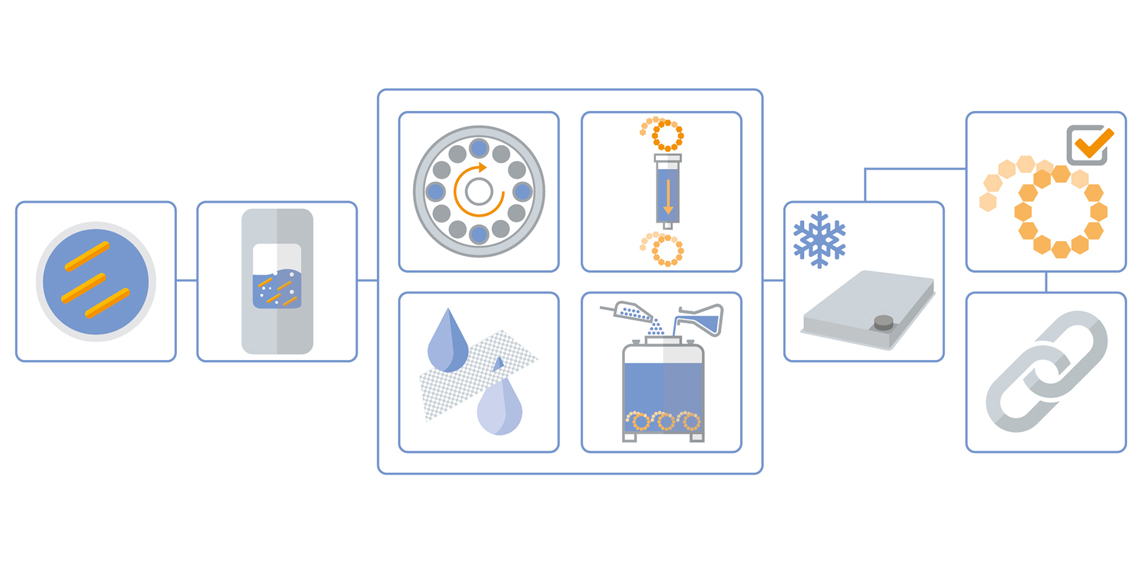
Inoculation & Precultures
The strain(s) for the production of polysaccharide(s) are provided by the customer for the initial production step.
Fermentation
Leveraging Wacker Biotech’s EMA/FDA approved manufacturing lines (fermenter capacities ranging from 250 L single-use to 1,500 L stainless steel reactors) allows customers to adjust the scale of GMP processes to meet the needs of each project and development phase.
Purification
Purification of the polysaccharide from the fermentation broth usually includes several steps, such as:
• Centrifugation of the pathogen after chemical/thermal inactivation
• Concentration of intermediate product by ultrafiltration and/or depth filtration
• Precipitation of the polysaccharide using inorganic salts and/or organic solvents (explosion-proof rooms and equipment required)
• Chromatography steps
Lyophilization
Conjugating the purified polysaccharide to a carrier protein involves dispensing the polysaccharides into LYOGUARD® trays for lyophilization. Bulk lyophilization runs of up to 65 L are supported.
Activation
The bulk lyophilized polysaccharides are activated through a chemical activation step.
Conjugation
In the final step, the activated polysaccharides are chemically conjugated to the carrier protein(s).
Product/Customer Spotlights
Vaxchora® for PaxVax
At our Amsterdam site, we manufactured Vaxchora®, a live, attenuated oral cholera vaccine for the commercial market for customer PaxVax (since acquired by Emergent BioSolutions). Oral vaccines are now the standard for helping to prevent cholera disease after injectable vaccines were discontinued in the 1970s when they were found to cause too many side effects.
Approved by the FDA in 2016, Vaxchora® is for those 2 to 64 years of age who are traveling to an area where the disease is endemic. It is the only cholera vaccine approved in the United States and has also been approved by the European Medicines Agency (EMA).
Vibrio cholerae is a species of Gram-negative, facultative anaerobe bacteria that frequently causes intense and fatal diarrhea and vomiting. Our state-of-the-art facilities were ideal to conduct the fermentation for this bacterium in a monoseptic, Biosafety Level 2 environment.
GBS Vaccine for MinervaX
Denmark-based MinervaX recently answered the call of the World Health Organization to develop a vaccine for Group B Streptococcus (GBS), a bacterium that can cause life-threatening infections in infants, pregnant women and older/at risk adults. It is linked to half a million pre-term births annually. Not only will a vaccine save lives, but it will also alleviate the need for excessive use of antibiotics, which can lead to antibiotic resistance.
Arising out of research conducted at Lund University, MinervaX’s lead vaccine candidate is a novel protein-only vaccine, based on fusions of highly immunogenic and proactive protein domains from selected surface proteins of GBS. Wacker Biotech will produce a supply of the active protein ingredient for clinical Phase III clinical testing. Our vaccines center of excellence in Amsterdam will also perform technology transfer, process characterization and process validation for later-stage commercial manufacturing.
"Wacker Biotech is a robust manufacturing partner with a strong track record in late clinical and commercial supply and we look forward to collaborating with the team ahead of commencing Phase III studies."
Click here for our vaccines brochures or read the press releases on some of our vaccine partnerships
Brochures
Links
MinervaX and Wacker Biotech Announce Manufacturing Collaboration for Prophylactic Vaccine Targeting Group B Streptococcus, September 14, 2024
view linkPandemic Preparedness: WACKER and CordenPharma Will Produce mRNA Vaccines for Germany When Needed, April 11, 2022
view linkCureVac and WACKER Sign Manufacturing Contract for CureVac’s COVID-19 Vaccine Candidate: CVnCoV, November 23, 2020
view linkBetter with WACKER
We look forward to bringing your biologics to life.