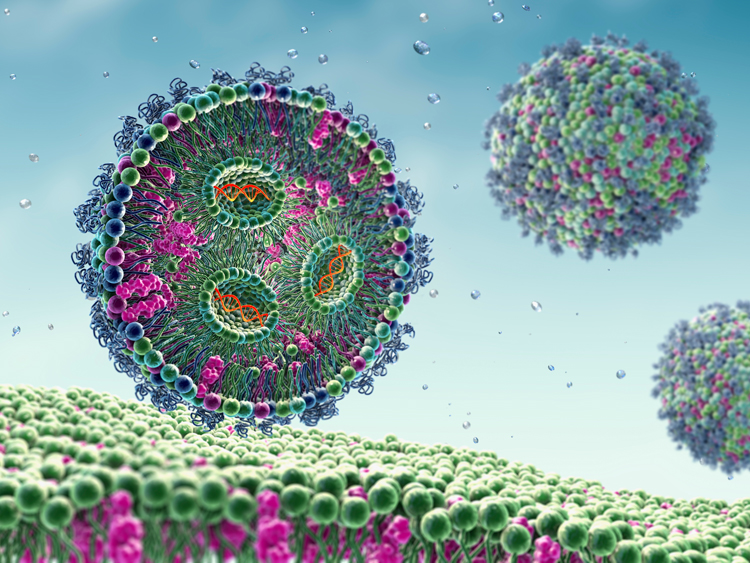
Lipid Nanoparticle Formulation (LNP)
Wacker Biotech is your expert partner for lipid nanoparticle (LNP) formulation. We can work with you to produce custom LNPs, or we can collaborate to build these on behalf of our clients via partner relationships, such as our relationship with CordenPharma.
The last step in the manufacturing chain for mRNA-based vaccines and advanced therapies, LNP formulation involves packaging the therapeutic cargo inside LNPs. The significant advantage of LNPs is their ability to deliver mRNA and other nucleic acid-based pharmaceuticals safely and effectively to the human body.
LNP Innovation and Expertise
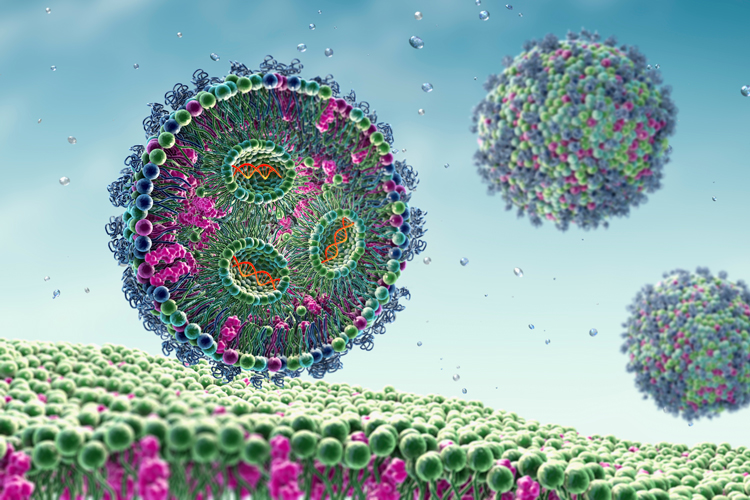
Wacker Biotech innovates in LNP formulation, which requires a delicate balance of different lipids and mixing phases.
A multi-component ethanol phase often contains:
An acidic aqueous T-mixer phase containing the mRNA, which is merged with the ethanol phase in a mixing process to yield the lipid nanoparticles.
LNP Capabilities
Our LNP capabilities for preclinical and clinical supply span the following areas:
Cell culture assays for functional LNP testing – covering multiple areas:
Key Benefits of Wacker Biotech’s LNP Capabilities
Ready to Partner with Us?
Discover how Wacker Biotech can transform your biopharmaceutical production. Contact us today to learn more about our innovative solutions and how we can help you achieve your goals. Reach out to our team for more information.
Product/Customer Spotlight
Pantherna Therapeutics
In April 2024, Wacker Biotech and Pantherna Therapeutics announced a partnership to produce the mRNA/LNP-based drug PAN004, a novel experimental therapeutic for acute respiratory distress syndrome (ARDS) targeting the Angiopoietin-1/2 system. Production of a clinical supply for dose range finding studies is expected to begin in 2025.
Pantherna and Wacker Biotech have been collaborating since 2022. As part of a joint research project, both companies initially developed a tailored manufacturing process for mRNA-based active ingredients, modifying WACKER’s own process. The plasmid-based template DNA, yield and critical parameters were all optimized by focusing on process scalability. The LNP manufacturing process based on Pantherna’s proprietary LNP formulation technology PTXΔLNP© was transferred from Pantherna to Wacker Biotech as part of this project. The resulting mRNA/LNP product was, in turn, evaluated by Pantherna in a cell culture model.
"We have established a very good collaborative R&D footing with WACKER and value its broad expertise in the production of mRNA active ingredients – expertise that we were able to use to turn our innovative technology into a product. WACKER plays a special role in the market, as it combines many years of experience in the toll manufacturing of complex biopharmaceuticals with the capabilities of its own Corporate R&D unit. We are delighted to now see our innovative mRNA active ingredient move from the laboratory to regulated production. This marks an important milestone for our company and opens a new chapter in our joint collaboration."
Click here to download our nucleic acid therapy brochures, white papers, plus related press releases, news stories and interviews.
Brochures
Wacker Biotech: Your CDMO for Advanced Therapies
DownloadWacker Biotech - Engage the Experts in pDNA, mRNA and LNP
DownloadPlasmid DNA GMP Manufacturing: Plug-and-Play Platform
DownloadWacker Biotech Contract Research Services
DownloadWacker Biotech | Antibody Conjugate Solutions
DownloadWacker Biotech | Cell & Gene Therapy Solutions
DownloadLinks
Our mRNA Competence Center in Halle and Interviews with our Experts
view linkWACKER and CordenPharma Enter Pandemic Readiness on 1 June 2024 After Successful Expansion and Qualification, June 19, 2024
view linkWACKER Opens mRNA Competence Center in Halle an der Saale, Germany, June 3, 2024
view linkPantherna and WACKER Intensify Collaboration in the Development and Production of mRNA Biopharmaceuticals, April 5, 2024
view linkEnlisting mRNA in the Fight against Cancer, October 18, 2022
view linkPandemic Preparedness: WACKER and CordenPharma Will Produce mRNA Vaccines for Germany When Needed, April 11, 2022
view linkCureVac and WACKER Sign Manufacturing Contract for CureVac’s COVID-19 Vaccine Candidate: CVnCoV, Nov 23, 2020
view linkBetter with WACKER
We look forward to bringing your biologics to life.