Expert Live Microbial Product Manufacturing
For more than 18 years, Wacker Biotech has been a pioneer in the development and manufacturing of Live Microbial Products (LMP). Our Amsterdam site is home to our LMP center of excellence, with expertise producing drug products containing live microbes, including injectable immunotherapies, topical medicines, and live attenuated vaccines.
LMP Innovation and Expertise
Customers benefit from a broad range of GMP scales fitting the need of each project and development phase. We offer EMA/FDA-approved manufacturing lines ranging from 250 L single use to 1500 L stainless steel.
You also benefit from our unique LIBATEC® platform combined with our extensive experience with a wide variety of bacteria, including wild-type, genetically modified, aerobic and anaerobic strains. These bacteria can be used for a broad range of LMPs, including the FDA-defined class of Live Biotherapeutic Products.
LMP Capabilities
Key Benefits of Wacker Biotech as Your LMP Partner
Learn More about Wacker Biotech’s LIBATEC® Platform for Live Microbial Process Development and GMP Production
Wacker Biotech’s proprietary LIBATEC® (Li ve Ba cterial Tec hnology) platform enables manufacturing without hold times – a critical necessity when working with live organisms – and maintains cell viability throughout the production process. The platform is also perfectly suited for a wide variety of live microbial strains and fermentation regimes for both aerobic and certain anaerobic organisms.
Our LMP Production Process
Click orange dots to learn more about each process step
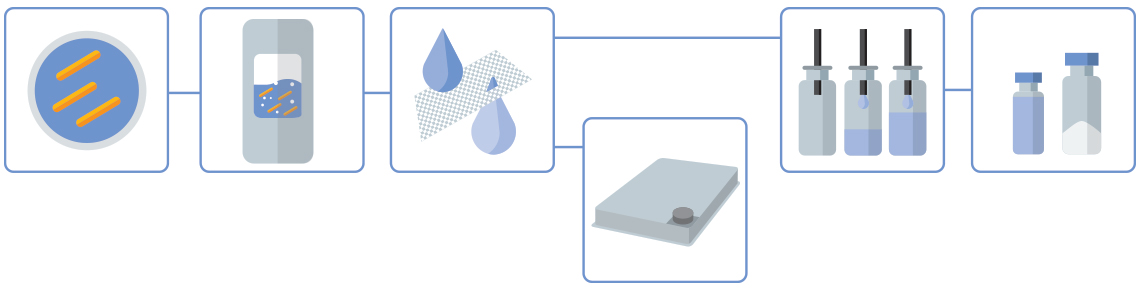
Inoculation & Precultures
The strains are provided by the customer.
Fermentation
Leveraging Wacker Biotech's EMA/FDA-approved manufacturing lines (ranging from 250 L single-use to 1500 L stainless-steel fermenters) allows customers to adjust the scale of GMP processes to meet the needs of each project and development phase.
Yield & TFF
An existing centrifugation step is usually replaced with an ultra-/micro-filtration step using tangential flow filtration (TFF). This is also a disposable technology and ensures the process remains closed and monoseptic at all stages.
Drug Substance
Bulk drug stubstances can be dispensed into trays and lyophilized. Bulk lyophilization runs up to 65 L are supported.
Drug Product
Aseptic filling operations are carried out within Wacker Biotech's GMP fill & finish facility, which includes both a formulation/preparation area and a principal filling area.
Liquid Formulation or Lyophilization
Drug products can be dispensed into vials. Batch fills are possible up to 20,000 units (DIN 2R to 20R) for both liquid and lyophilized formulations.
Customer/Product Spotlights
Aurealis Therapeutics
Aurealis Therapeutics AG approached Wacker Biotech for process development, scale-up and GMP manufacturing of AUP1602-C, their lead candidate for chronic wound applications. Genetically modified lactic bacteria are administered topically to promote tissue regeneration in patients suffering from non-healing chronic wounds. Manufacturing under monoseptic conditions was required because the product will be in direct contact with human tissue.
The process was successfully transferred, adapted for monoseptic manufacturing under GMP conditions and scaled up. Subsequent manufacturing was performed under monoseptic GMP conditions using Wacker Biotech’s 250 L single-use bioreactor, followed by aseptic filling into ~4,000 vials per batch. The process developed was shown to be highly robust, yielding cells that are resistant to processing, hold times and storage at low temperatures. Cell viability of the resulting drug product met the defined criteria (1010 – 1011 colony forming units/mL). Wacker Biotech also performed setup, development and validation of the required analytical assays for product testing. The drug product was successfully released for use in phase I clinical trials, and a Phase II trial is now planned.
"We are very pleased with the high technical competence, reliability and support of the Wacker team to bring our lead candidate into the clinic as planned – Wacker is a true partner in this project."
Prokarium
For London, UK-based Prokarium, a pioneer in the field of microbial immunotherapy, Wacker Biotech is conducting GMP manufacturing of an oral biopharmaceutical for bladder cancer. Prokarium’s pipeline is leveraging the most recent advances in cancer immunology to take immuno-oncology to the next level. In particular, it uses genetically modified salmonella as a delivery mechanism for delivering anti-tumor payloads to bladder cells.
"We are very pleased to work with Wacker Biotech given their deep expertise with live microbial products and GMP production."
Ready to Partner with Us?
Discover how Wacker Biotech can transform your biopharmaceutical production. Contact us today to learn more about our innovative solutions and how we can help you achieve your goals. Reach out to our team for more information.
Better with WACKER
We look forward to bringing your biologics to life.