Mar 01, 2018 Read time: approx. MinutesMinute
Key Component Lithium-Ion Battery
Lithium-ion batteries are rechargeable battery systems that use lithium ions as mobile positive charge carriers in their cells. The cells contain two electrodes that can reversibly store the lithium ions and an electrolyte in which the lithium ions can be transported between the electrodes. A thin membrane, known as a separator, is positioned between the electrodes. This membrane is an electrical insulator, but is permeable to lithium ions. Chemical reactions take place in both electrode materials – spatially separated from each other – and this generates an electric voltage between the electrodes.
Current traction-battery production often uses cells with graphite anodes and a lithium-nickel-manganese-cobalt oxide (abbreviated to NMC) for the cathodes. The electrode materials are present as thin layers on metal foils which act as current collectors, transferring electrons that are flowing in or out. The electrolyte is a solution of a conducting salt that contains lithium ions (lithium hexafluorophosphate) in a mixture of organic carbonates.

For more information on the topic of electromobility read our article “An Energizing Lead”
Such NMC-graphite battery cells achieve both a high specific energy and a high power density, and can therefore store large amounts of energy per kilogram and rapidly release the stored energy. Specific energies of up to 260 Watt hours per kilogram for these battery cells are technically feasible, but not yet common in the industry. If an electric consumer is connected to the charged cell, the anode donates electrons to its current collector, releasing the stored lithium ions. The electrons flow through the external consumer circuit, where they do their work, before flowing on to the cathode, which accepts the electrons via its current collector. At the same time, the lithium ions released from the anode’s graphite material move through the electrolyte to the cathode and are stored there. The entire procedure occurs in reverse when the battery is being charged.
A certain amount of electrolyte decomposes on the anode surface during the very first charge that takes place when the battery is being manufactured. The decomposition products, which contain lithium, form a solid film on the anode. This surface film, known as SEI (solid electrolyte interphase), is permeable to lithium ions and protects the anode from further decomposition. Parts of the SEI can be damaged due to various battery cell issues such as deep discharging or even high temperatures. Although the SEI is formed again during the next charge, the damage leads to battery-capacity reduction because of the irreversible loss of lithium ions in the electrolyte: the battery can no longer store as much charge as before.
Overcharging the battery is also damaging, as it can cause the release of so many lithium ions from the cathode material that its structure is damaged. There is also the danger of metallic lithium being deposited on the anode in the form of pointed, branched crystals, known as dendrites. These electrically conductive lithium dendrites can penetrate the separator and cause an internal short circuit.
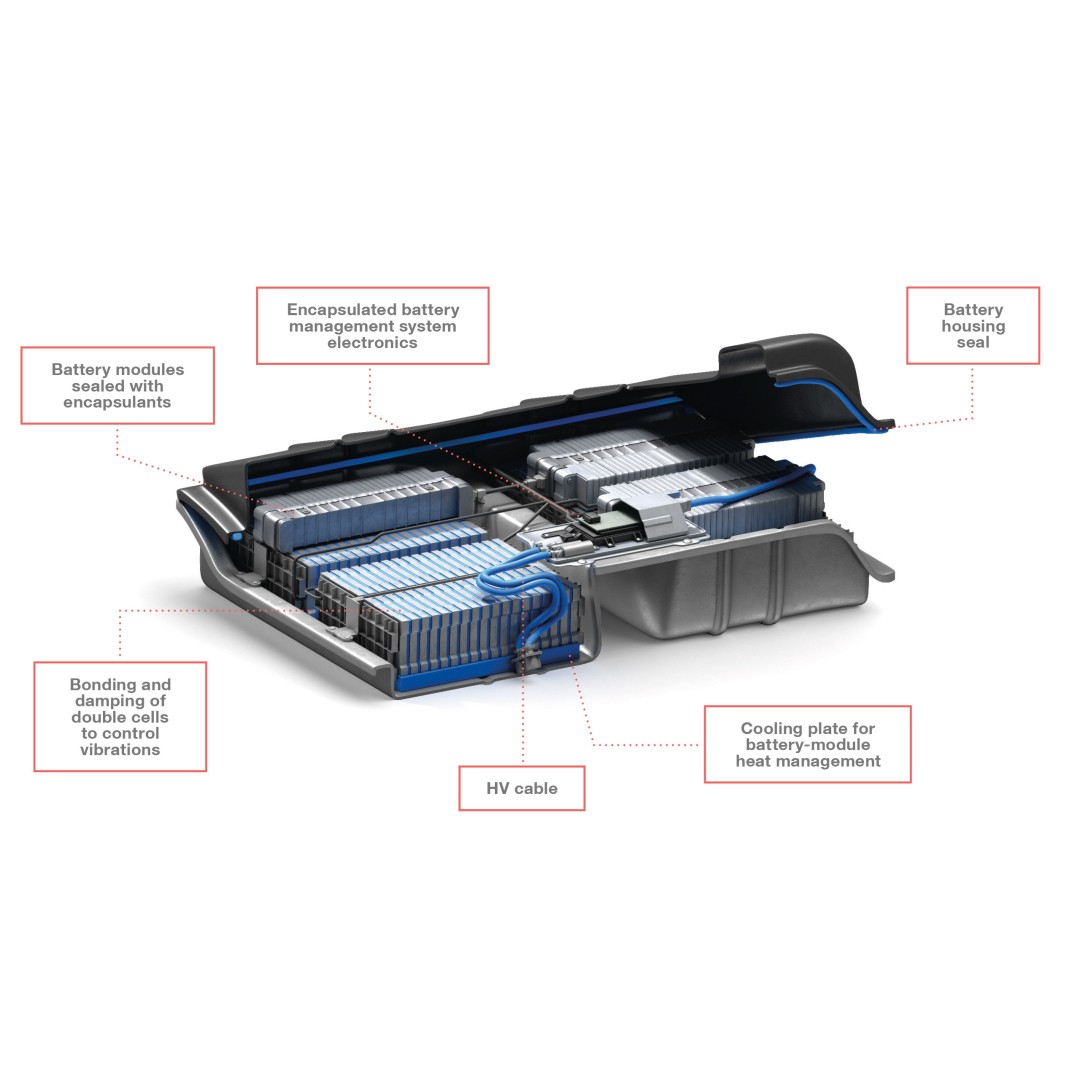
Numerous silicone rubber products (shown in blue) are used in a typical traction battery.
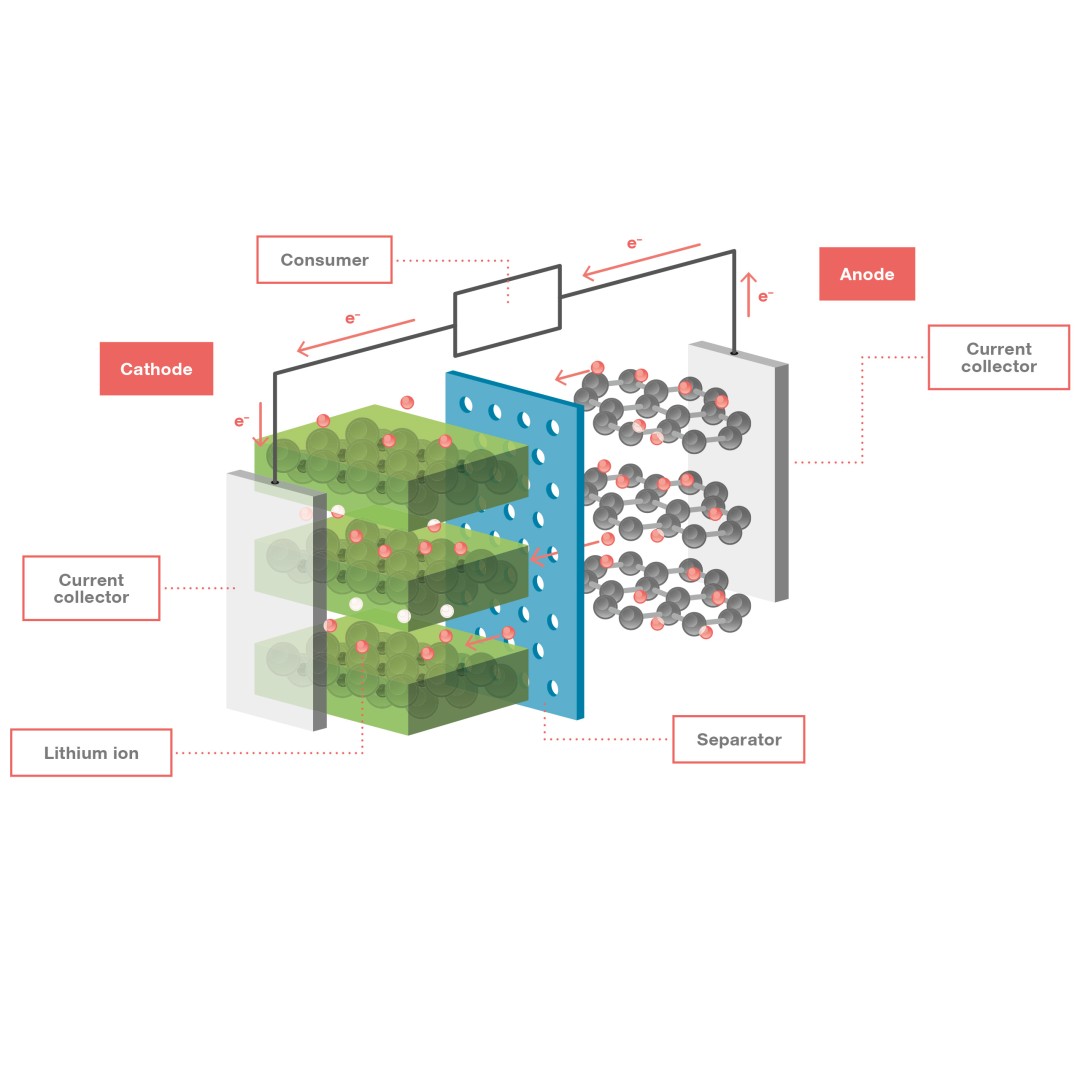
Configuration of a lithium-ion battery and how it works: The anode material releases stored lithium ions when the cell is discharged, donating electrons (e–) to the current collector. The released lithium ions move through the electrolyte to the cathode, pass through the separator and are stored in the cathode material. In the cell, the anode forms the negative terminal and the cathode the positive terminal.
Batteries need to be temperature controlled
The colder the battery is, the more viscous the electrolyte becomes and this increasingly hinders the movement of lithium ions. At low temperatures, there is a danger of overpotential during charging causing lithium to be deposited in metallic form instead of being stored in the graphite.
High temperatures accelerate chemical reactions. This is particularly noticeable as undesirable aging and degradation processes in lithium-ion batteries. Temperatures above 45 °C can cause a lithium-ion battery to age rapidly, while the conducting salt reaches its thermal stability limit at 60 °C. The optimum temperature is 25 °C.
The battery therefore needs to be temperature controlled – it must not get too hot or too cold during operation. In practice, the traction battery is maintained at operating temperatures between 0 and 45 °C. The newly developed silicone gap fillers from WACKER support heat transfer between the battery modules and the temperature-control system, for instance in the cooling plate located in the lower housing shell and through which coolant flows.
Mr. Dr. Klaus Angermaier
Global Business Development
WACKER SILICONES
+49 89 6279-1453
dr.klaus.angermaier@wacker.com
