pDNA / mRNA / LNP
Wacker Biotech is your CDMO partner and resource for advanced therapies, including messenger RNA (mRNA), plasmid DNA (pDNA) and lipid nanoparticle formulations (LNP’s). A great team you can trust!
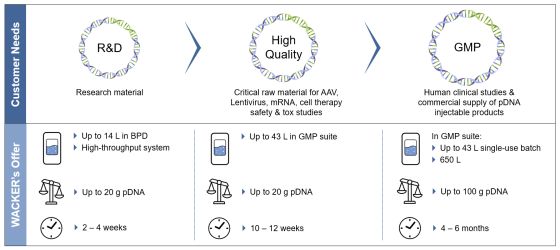
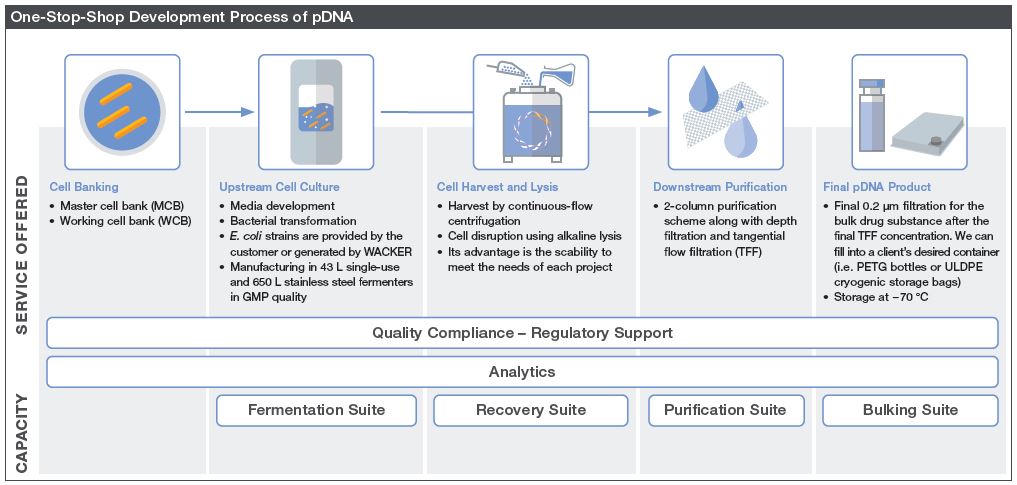
Our customers benefit from Wacker Biotech’s R&D and GMP solutions as a one-stop-shop for the development and GMP manufacturing of mRNA / pDNA at scales to meet your clinical and commercial needs.
During the Corona pandemic, Wacker Biotech jump-started mRNA manufacturing in Amsterdam, The Netherlands, with large-scale production capabilities for mRNA process transfers, and is now one of the few CDMOs with hands-on GMP manufacturing experience of mRNA-based therapies up to process validation. Now, we are taking this to the next level! In Halle, Germany, we are building a brand-new mRNA competence center with GMP capabilities for pDNA, mRNA and LNP manufacturing – all under one roof in the heart of Germany. The new plant is part of the German Pandemic Preparedness Initiative, but half of the capacity is available to customers from early clinical to commercial. The expansion will be completed by Q1/Q2 2024. Our development labs are already up and running to support technology transfers, process development or R&D supply. Wacker Biotech is part of a new synergistic network formed during the pandemic – one of these partners is CordenPharma. The nucleic acid experience of Wacker Biotech combined with the lipid expertise of CordenPharma ensures the best possible results for each of our customers’ mRNA projects.