Biotechnology
WACKER Building mRNA Competence Center in Halle
Oct 18, 2022 Read time: approx. MinutesMinute
Enlisting mRNA in the Fight against Cancer
Vaccines based on mRNA protected humanity against a worst-case scenario during the Covid-19 pandemic. The mRNA therapeutics not only protect against virus infections, they also have great potential for tumor therapy. WACKER is now building a new mRNA competence center in Halle to complement its three existing sites in Germany, the Netherlands and the US (California).

Bacteria at Wacker Biotech in Halle produce pharmaceutical proteins in stainless steel tanks known as fermenters. The site is being expanded into an mRNA competence center, scheduled to start up in 2024 and featuring a new technology.
Until just a few years ago, mRNA therapeutics were only familiar to specialists. Today, most people are at least familiar with the term. The reason? Coronavirus. “The high demand for new and, above all, rapidly available vaccines has shown the potential of this technology. The biopharmaceutical sector has experienced a major boost,” said Dr. Guido Seidel, managing director at Wacker Biotech. He compared the situation with the amazing rise of antibodies just a few decades ago: “There were a lot of skeptics in the 1980s who dismissed any potential for this technology. Today, highly specific antibodies are used in standard therapies for numerous diseases.”

The production capacity will more than triple thanks to the future mRNA competence center in Halle – proposed design shown here.
The same point, he explained, had now been reached for mRNA technology: “Proof of concept” has been achieved. Whether this technology works for other indications still has to be demonstrated. Seidel is confident: “We will be able to treat or even prevent numerous diseases in 20, 30 years with mRNA-based medication.”
More research, more demand, more business – WACKER is moving with the current rise in demand and has decided to expand the Wacker Biotech GmbH site in Halle to become an mRNA competence center. The WACKER Group focuses all its biopharmaceutical activities within its Wacker Biotech subsidiary. Ground was broken at the beginning of July 2022. The new production building at the Halle site in Saxony-Anhalt will be up and running within two years, with some 200 people working there. Plans are for four production lines which will exclusively manufacture mRNA biopharmaceuticals. This means that Wacker Biotech will greatly increase its production capacity based on its current site capacities in Jena and Halle (Germany), and in Amsterdam (NL) and San Diego (USA). “It is no longer possible to conceive of medical biotechnology not being used in patient treatment. Almost half of all newly approved medicines are now biopharmaceuticals,” Seidel underlined. According to experts, the percentage of mRNA therapeutics will continue to rise.
“It is no longer possible to conceive of medical biotechnology not being used in patient treatment.”
Dr. Guido Seidel, managing director of Wacker Biotech


New Competence Center for mRNA Active Ingredients in Halle
The start of the Weinberg campus expansion was marked with a symbolic groundbreaking ceremony. Over the coming months, a competence center for active ingredients based on messenger ribonucleic acid (mRNA) will take shape here. Numerous guests from politics and business attended the ceremony: (from left) Michael Zorn from Saxony-Anhalt’s State Administration Office; Egbert Geier, Mayor of Halle; Melanie Käsmarker, co-managing director of Wacker Biotech GmbH; Sven Schulze, Saxony-Anhalt’s Minister of Economic Affairs; Christian Hartel, president & CEO of Wacker Chemie AG; Armin Willingmann, Saxony-Anhalt’s Minister of Science, Energy, Climate Protection and Environment; Wolfgang Büchele, CEO of Exyte; Guido Seidel, co-managing director of Wacker Biotech GmbH; Ulf-Marten Schmieder, managing director of the Weinberg Campus technology park; and Joachim Bug, deputy director of ZEPAI (Center for Pandemic Vaccines and Therapeutics).
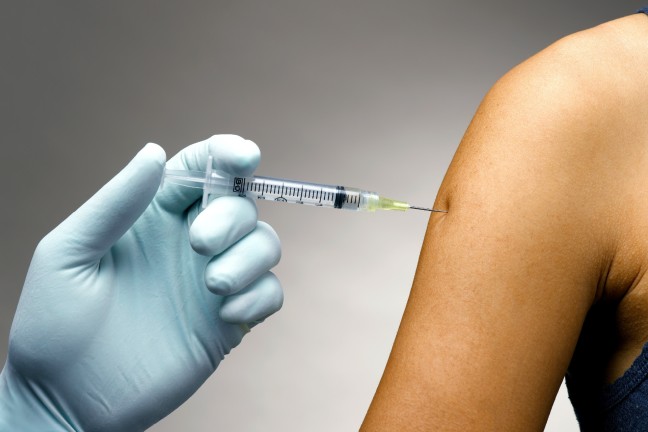
mRNA-based vaccines require special handling and may only be thawed shortly before being administered.
Detailed Blueprint
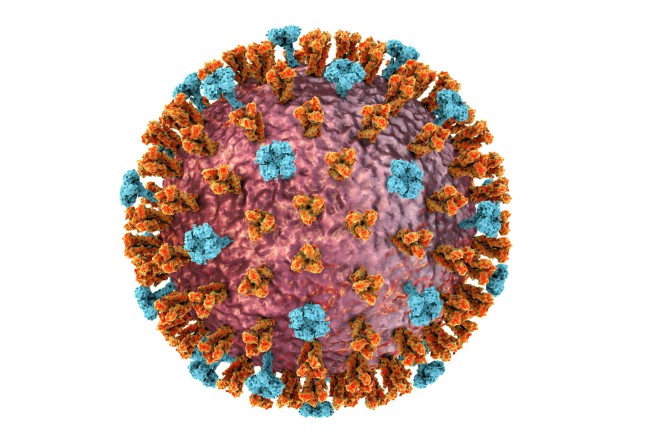
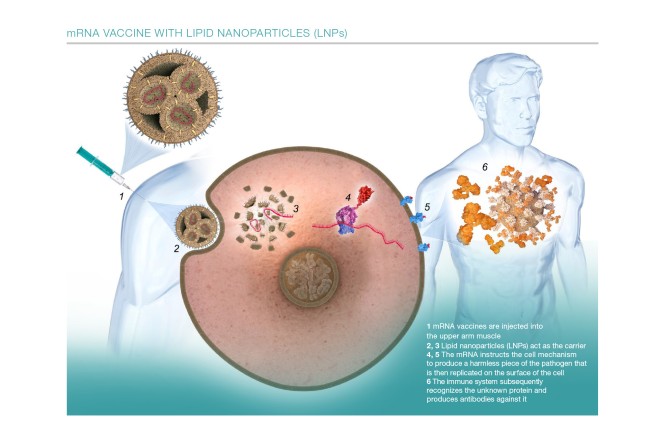
The foundation stone was laid by vaccine development during the current pandemic. In just a few months, numerous biotechnology companies developed vaccines against the SARS-CoV-2 virus. The main constituent is mRNA. The abbreviation stands for messenger ribonucleic acid (RNA). While previous vaccines usually consist of virus particles or fragments of such particles, mRNA vaccines take a slightly different path: they consist of a detailed blueprint of the special proteins in the pathogen, also known as antigens. In the coronavirus, for instance, this is the spike protein that protrudes from the surface of the virus, providing it with a striking appearance. Packed in a lipid envelope, the genetic message is delivered into the muscle cells at the injection point. The cells then build the coronavirus protein, present it to the immune system, which recognizes the antigen as an intruder and starts producing antibodies against it. Should the body one day be infected with the actual pathogen, it is then suitably equipped and can effectively combat the intruder.
In order to understand what is so unusual about mRNA, you need to look inside the cell nucleus. This is where the DNA containing human genetic information is stored – all the information needed for a blueprint of the human body. Each gene codes for a protein, and these genes determine the characteristics of every life form. It has been 60 years since it became clear that cells produce a copy of the genes in order to translate the information in a protein. Whenever the body requires a specific protein, the required gene is translated in an mRNA. The mRNA transfers the blueprint for the protein from the cell nucleus to the ribosomes. The ribosomes in the cells then produce the required protein. The DNA remains protected in the cell nucleus.
Large Volumes, Rapid Production
It has now become possible for the first time to use these mRNA vaccines to create therapeutic agents based on mRNA on a large scale. Over 12 billion vaccinations worldwide up until June 2022 have shown that mRNA vaccines work effectively, are well tolerated and can be efficiently produced. Many were astounded at how rapidly the appropriate vaccinations became available in such huge volumes. This rapid development was made possible because many building blocks for mRNA vaccine had already been created, emphasized Seidel. He is himself a biochemist and has been familiar with the research and production of biopharmaceuticals for more than 20 years: “That production ramped up so fast is because the spike sequence mRNA could be conceived, designed and synthesized so rapidly, relatively speaking, compared to cancer therapeutics that companies have been researching so intensively to date.”
That this experience has resulted in numerous ideas for further vaccines based on the same principle is a logical conclusion: the US biotech company Moderna is working on, among other things, a quadruple vaccine against the influenza virus. The company even intends to create a form of “autumn vaccine” that combines different vaccines against various respiratory diseases such as Covid-19, influenza and respiratory syncytial virus (RSV).
There is a lot to suggest that mRNA therapeutics will also find a use in the future for other indications. Biontech founder Ugur Sahin, for example, believes that his company will introduce multiple products to combat cancer and infectious diseases over the next three to five years. “You can use mRNA anywhere in the body where proteins are functioning incorrectly or where proteins are needed to activate the immune system. The coronavirus vaccine is one example – and so is the fight against cancer,” said Seidel. Possible application areas for mRNA therapeutics could be autoimmune diseases, cardiovascular diseases and in regenerative medicine.
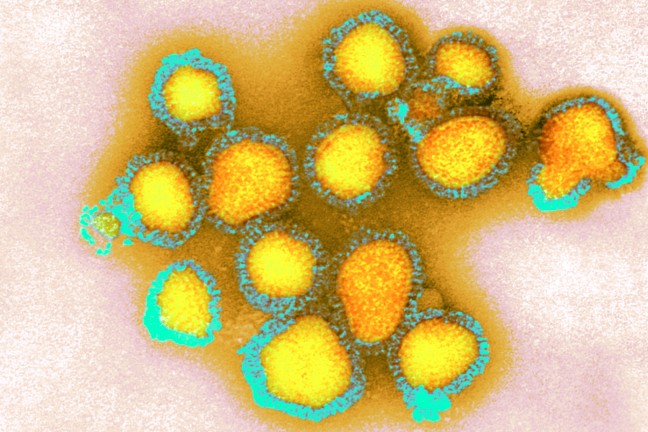
The H3N2 subtype of the influenza A virus was responsible for the Hong Kong flu at the end of the 1960s – a pandemic that was variously estimated to be responsible for between one and four million deaths.
“mRNA can be used anywhere where proteins are malfunctioning in the body.”
Dr. Guido Seidel, managing director of Wacker Biotech
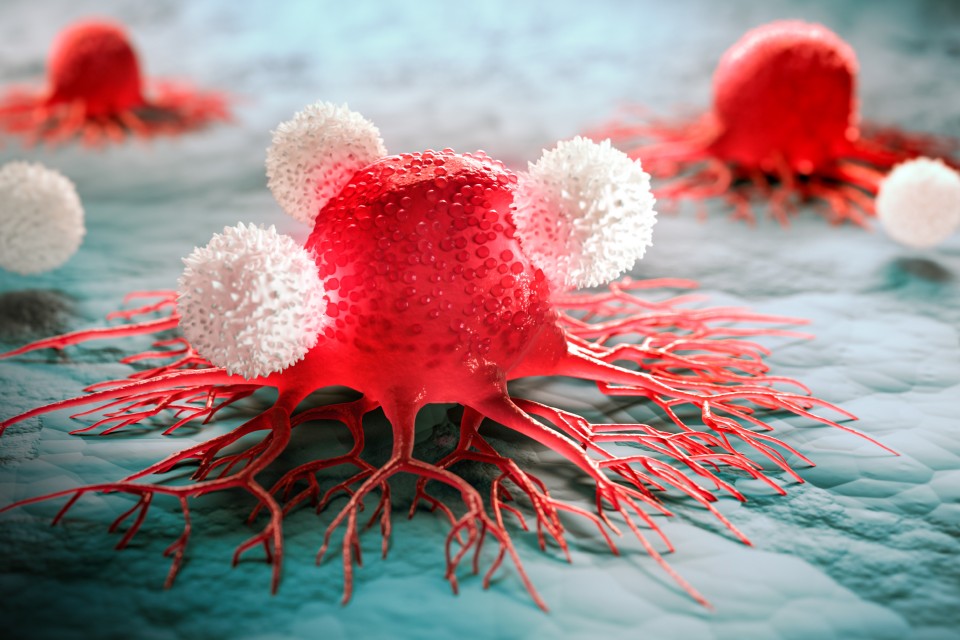
Developers are placing a lot of hope in cancer treatment in particular. The challenge for a cancer vaccination is that cancer cells often disguise themselves and therefore avoid being destroyed by the immune system. The mRNA vaccine is intended to induce the immune system to recognize tumor cells as “foreign” again and effectively fight them. The mRNA vaccine therefore contains the blueprints of important components of individual tumors, for instance a surface protein. The resulting protein or peptide then activates the immune system, which combats the cancer cells. “The key step here is to rapidly produce a suitable active ingredient from the tumor data,” said Seidel, adding that mRNA was predestined for this as it can be relatively rapidly designed and produced.
As is often the case during the development of new medicines, numerous actors are essential: while pharmaceutical companies drive research forward, contract manufacturing organizations such as Wacker Biotech ensure that the new active ingredients can be produced in large quantities. As a contract development and manufacturing organization (CDMO), the company produces active ingredients for the market and clinical testing on behalf of pharmaceutical and biotech companies. “The rapid success in vaccine production would hardly be possible without companies like us,” said Seidel. As a full service producer of biologics, Wacker Biotech has 20 years of experience in the sector of microbial systems. Core competencies include the production of pharmaceutical active ingredients, live bacteria and vaccines – lately also based on mRNA. Wacker Biotech has built up an international portfolio of customers over the years. Business is growing steadily.

From development to dispensing: at Wacker Biotech’s Amsterdam site, active ingredients can be dispensed into their final packaging for shipment to customers. The vials then undergo one last quality control step.

As a contract manufacturer, Wacker Biotech US Inc. in San Diego operates a specialized fermentation line for manufacturing and purifying plasmid DNA.
Potential Recognized Early On
The biotech company recognized the potential in mRNA therapeutics early on: “We have been intensively expanding our expertise and production capacities for mRNA therapeutics since 2018,” said Seidel. The various sites cover different specialist areas of the manufacturing processes: in Amsterdam for example, they know how to package the highly sensitive molecules into tiny lipid spheres. Plasmid DNA is produced in San Diego as the raw material for mRNA therapeutics.
WACKER eventually decided to focus all these mRNA competencies at one site: “Together with our partner, CordenPharma, our Halle site will, going forward, cover the complete manufacturing process for mRNA active ingredients,” said Seidel. While Wacker Biotech takes over the production of mRNA derived from plasmid DNA and the preparation of the mRNA active ingredient with lipid nanoparticles (LNPs), CordenPharma is to produce the lipids for the LNP preparation and take over the sterile filling and packaging of the mRNA vaccine.
Four new production lines will be set up in Halle for this purpose. Approximately half of the production capacity will be reserved for the German Government within the framework of its pandemic preparedness plan contracts. Seidel explained that the concept of a ‘warm facility’ would be followed here: “Systems will be operational and be maintained in a state of full readiness. If we receive the order from the German Government to produce mRNA vaccine, we can start production within a very short time.” The aim is to be optimally prepared for a future pandemic and thus avoid any supply bottlenecks like those that occurred at the start of the coronavirus pandemic. Seidel is very proud that WACKER will be producing mRNA vaccines for the German Government if needed: “We are very happy that we have invested in this technology over the years – and are now playing in the front row.”
If necessary, Wacker Biotech and CordenPharma can supply Germany with 80 million vaccine doses per year. The plan is to use the remaining production capacity in Halle to contract-manufacture mRNA therapeutics. The first products will emerge from the Halle plant beginning in mid-2024. Wacker Biotech is already putting out feelers to customers who could in the future produce in Halle. Seidel’s dream? To be present when the new technology will also be able to heal cancers in the future. “It is so exciting to imagine that we will one day be able to contribute towards improving the fight against tumors in a truly wide range of cancer indications, and to heal patients in a highly specific manner.”
Mrs. Manuela Dollinger
Manager Media Relations & Informations
+49 89 6279-1629
manuela.dollinger@wacker.com